Still valid after 1 year and a half! Hemophilia gene therapy shows excellent data
Still valid after 1 year and a half! Hemophilia gene therapy shows excellent data
December 12, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];At the annual meeting of the American Society of Hematology (ASH), BioMarin published the latest data on its hemophilia A gene therapy valoctocogene roxaparvovec (formerly known as BMN 270). Its excellent efficacy became one of the focuses of the conference.

Hemophilia A is a serious blood disease. The patient lacks the key factor VIII, so it can't coagulate effectively. If it is slightly injured, it will bleed and there is a risk of life-threatening. For those with severe hemophilia, their muscles and joints will spontaneously bleed, causing great pain and inconvenience to them. Even more unfortunately, although hemophilia A is a genetic disease, it is estimated that one third of patients are caused by spontaneous mutations. This disease area therefore has enormous medical needs. Currently, the standard treatment for hemophilia A is the infusion of prophylactic factor VIII three times a week. This therapy not only requires frequent patient treatment, but also does not completely eliminate the patient's bleeding hazard. Patients and doctors are also looking forward to the emergence of new therapies.
Valoctocogene roxaparvovec from BioMarin is a gene therapy that is expected to treat hemophilia A. Using an adeno-associated virus vector, this therapy has the potential to bring the blood levels of Factor VIII in the patient to near normal levels and restore normal blood clotting capacity.
At this year's ASH annual meeting, BioMarin's clinical data supports the potential of this therapy. In a phase 1/2 clinical trial, patients were divided into two groups and received two doses of 4e13 vg/kg and 6e13 vg/kg, respectively. In the previous group, the follow-up time of the three patients reached 48 weeks. Their Factor VIII levels approached or reached normal values ​​with a median and mean of 49%. After 4 weeks of treatment, their median annual bleeding events were zero and factor VIII activity was increased by more than 5%. Their average annual bleeding event was 0.6, and factor VIII was used on average 2.0 times a year.
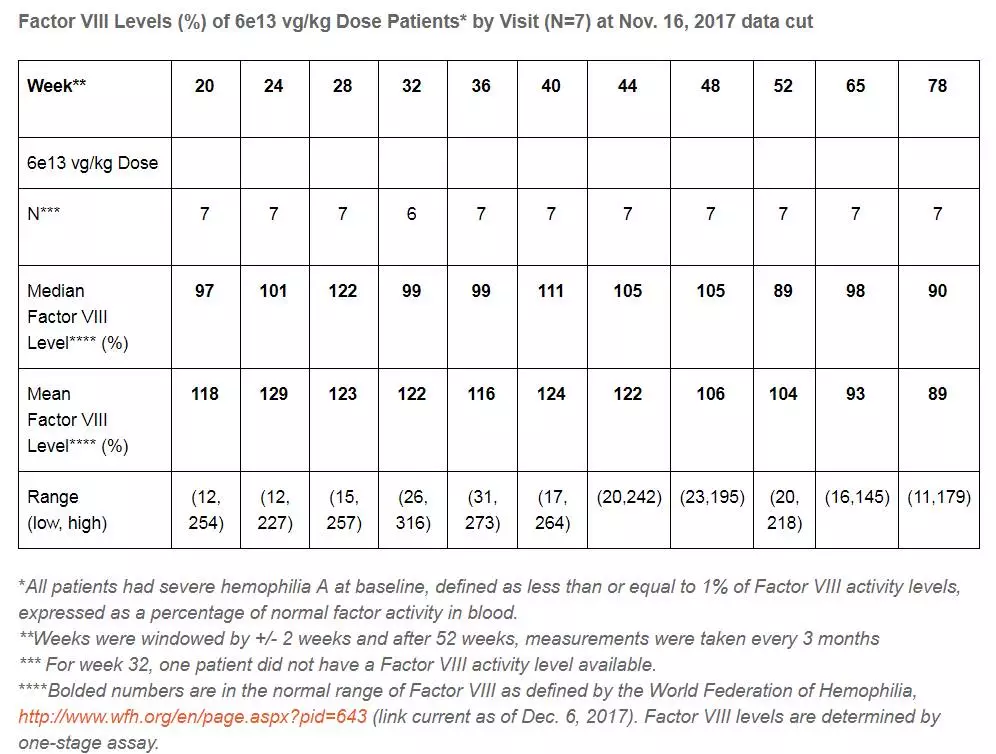
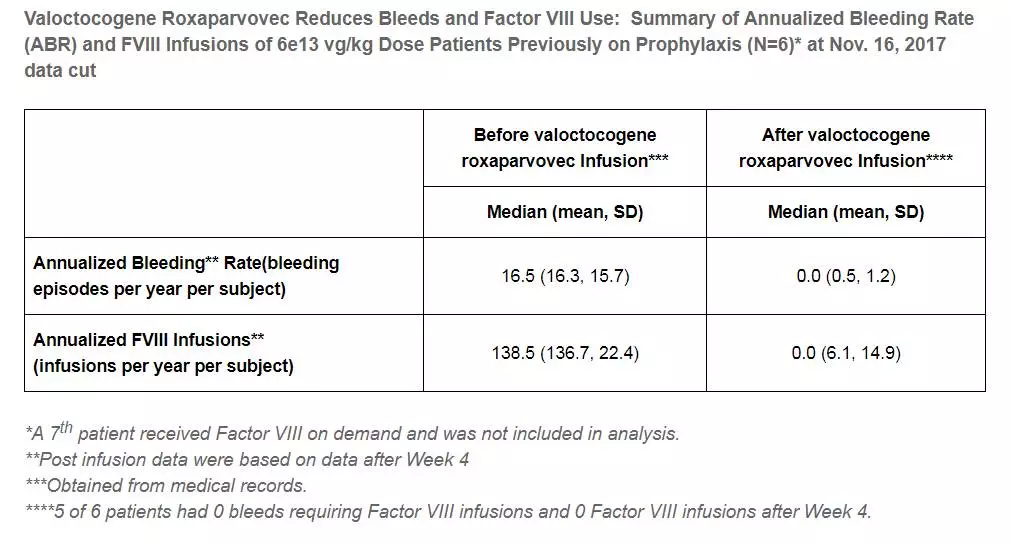
â–²The data of this therapy high dose group (Source: BioMarin official website)
In the high-dose group, valoctocogene roxaparvovec resulted in a higher level of factor VIII - after 78 weeks of treatment, the median factor VIII level was 90% and the mean factor VIII level was 89%. After 4 weeks of treatment, the number of median annual bleeding events was also 0, the average number of annual bleeding events was 0.5, and the number of factor VIII used was 6.1 per year. Some data from the high-dose group were also published last week in the New England Journal of Medicine.
“The convergence of innovative medicines and advanced therapies has brought unprecedented opportunities for hemophilia to improve their prognosis for today's and tomorrow's patients,†said Dr. Hank Fuchs, President of Global Development at BioMarin. “We are very excited to see that Today's data indicate that a valoctocogene roxaparvovec infusion has the potential to eliminate bleeding and eliminate the need for exogenous factor VIII infusion, allowing factor VIII levels in patients with severe hemophilia A to return to normal range. Its safety is also Equally acceptable. We are entering a new era of treatment for severe hemophilia. We look forward to bringing this innovative medical platform to patients."
We congratulate BioMarin for its outstanding achievements and wish that innovative gene therapy will be available as soon as possible for the benefit of patients!
Reference materials:
[1] BioMarin Highlights New Results for Gene Therapy Valoctocogene Roxaparvovec at the 2017 American Society of Hemophilia (ASH) Meeting
[2] BioMarin Provides 1.5 years of Clinical Data for Valoctocogene Roxaparvovec Gene Therapy for Severe Hemophilia A at 59th American Society of Hematology (ASH) Annual Meeting Concurrent with NEJM Publication
[3] BioMarin Official Website
Covid-19 Test Kit,Covid-19 Antigen Test,Covid-19 Antigen Rapid Test,Covid-19 Antigen Detection Kit
Hangzhou DIAN Biotechnology Co., Ltd. , https://www.dianbiotech.com