Effects of Immune Status on Nutritional Metabolism and Nutritional Requirements of Pigs
Immune System Activation Leads to Changes in Animal Body Metabolism
The activation of the immune system will increase the level of the corresponding antibodies, accelerate the proliferation of lymphocytes, increase the level of cytokines, and so on, so as to effectively resist the damage of animal antigens to the animals. However, the activation of the immune system has also brought about a significant negative effect, causing a series of behavioral and metabolic changes in animals. Typical symptoms of behavioral changes are animal fever, anorexia, decreased feed intake and growth rate, poor feed conversion rates, and animals in a clinically stressed state called "immune stress." Metabolic changes are the body's transfer of nutrients that are used for growth and skeletal muscle deposition to highly activated immune systems to fight disease.
Effect of immune system activation on pig production performance
The growth rate and body composition of a particular genotype pig depend to some extent on its health level. Reducing the activation of the immune system in pigs can increase pig feed intake, growth rate, and feed conversion efficiency. In addition, pig carcass composition can be altered so that pigs deposit more muscle or body protein and deposit as little fat tissue as possible.
Activation of the immune system can also affect the body condition and lactation capacity of nursing sows. The reason that high immune system activation affects sow's lactation is that cytokines inhibit the release of sow's milk producing hormones (such as GH, IGF-1, and prolactin) (Bauman and Vernon, 1993), thereby reducing GH, IGF in blood circulation -1 (Fan et al., 1995) and prolactin (Chao et al., 1994). There have been few reports on the effect of the level of immune system activation on the performance of lactating sows. Sauber et al. (1999) showed that a high level of immune system activation results in a 10% reduction in sow feed intake, but has little effect on sow weight loss, but also has a certain impact on milk composition; high immune system activation The milk protein content was reduced, but had no effect on milk fat content; eventually, the total milk production and milk protein production were reduced, but there was no effect on milk fat production. The decline in lactating ability of the sow eventually affected the growth performance of the suckling pig, which reduced the litter weight gain by 14% but did not affect the number of weaned piglets. The effect of immune system activation on the performance of pregnant sows has not been studied.
Effect of Immune System Activation on Nutrient Requirements of Pigs
At present, there are few studies on the nutrient utilization efficiency of pigs under different immune system activation conditions. Only Williams et al. (1997a,b) studied the energy and utilization efficiency of lysine. The effect of immune system activation on the utilization efficiency of other nutrients remains to be further studied.
Studies by Williams et al. (1997a) showed that different levels of immune system activation have no effect on the efficiency of the maintenance of energy requirements and metabolic energy of pigs for body protein and body fat deposition. Williams et al. (1997b) reported that the efficiency of lysine for body nitrogen deposition is similar under different immune system activation states.
Research on the nutritional requirements of pigs for different immune system activation states focuses on the study of amino acid requirements, especially on lysine requirements. Due to the low immune system activated pigs' muscle tissue growth ability was higher in the immune system activated pigs.
In production practice, the growth rate of animals is not constant. Between several consecutive rapid growth periods, the slow growth phase induced by immune system stress appears from time to time, and thus the requirement of nutrients presents a phase change. It can generally be divided into three phases: (1) the incubation period of immune stress, and the actual requirement of nutrients at this stage is equivalent to the recommendation of the NRC; (2) immune stress period, due to the abnormally active immune system, animal feed intake and growth. The speed is reduced, so the demand for nutrients is lower than the normal requirement. At present, researches on the effects of immune stress on nutritional requirements are concentrated in this stage; (3) Compensatory growth period after stress, at which the organism successfully excretes invading microorganisms, and the body compensates for growth. Higher than recommended by NRC. Compensatory growth nutritional needs after stress have not been studied.
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan, Risedronate Sodium, Atazanavir, Saxagliptin, Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising pharmaceutical intermediates manufacturers, chemical intermediates and bulk drug intermediates suppliers. Our consistent supply, quality products and dedication towards clients have opened up many international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
|
PRODUCT NAME |
CAS NUMBER |
SPEVIFICATION |
|
Azithromycin |
117772-70-0 |
BEP |
|
Cefpirome Sulphate sterile |
84957-29-9 |
USP JP16 |
|
Ceftriaxone Sodium (Sterile) |
104376-79-6 |
USP31 |
|
Cefotaxime |
64485-93-4 |
USP30 |
|
Ciprofloxacin HCL |
85721-33-1 |
USP/BP |
|
Gentamicin sulphate |
1405-41-0 |
BP |
|
Levofloxacin |
100986-85-4 |
USP27 |
|
Lincomycin Hydrochloride |
859-18-7 |
EP6.0 |
|
Moxifloxacin Hydrochloride |
186826-86-8 |
USP31 |
|
Tigecycline |
220620-09-7 |
USP |
|
Linezolid |
165800-03-3 |
EP |
|
Dexamethasone |
50-02-2 |
USP/BP/EP |
|
Methylprednisolone |
83-43-2 |
USP/BP/EP |
|
Dexketoprofen trometamol |
156604-79-4 |
BP2008 |
|
Ibuprofen |
15687-27-1 |
BP |
|
Metamizol |
68-89-3 |
DAB |
|
Sulindac |
38194-50-2 |
USP/BP/EP |
|
Naproxcinod |
163133-43-5 |
USP28 |
|
Tripelennamine Hydrochloride |
154-69-8 |
USP28 |
|
Itraconazole |
84625-61-6 |
USP/BP |
|
Cytarabine |
147-94-4 |
USP31 |
|
Leucovorin Calcium |
1492-18-8 |
USP32 |
|
Valsartan |
137862-53-4 |
USP30 |
|
Telmisartan |
144701-48-4 |
USP31 |
|
Rosuvastatin Calcium |
147098-20-2 |
USP/BP |
|
Pitavastatin Calcium |
147526-32-7 |
USP/BP |
|
Fluvastatin |
93957-54-1 |
USP31 |
|
Vinpocetine |
42971-09-5 |
EP6.0 |
|
Atazanavir |
198904-31-3 |
BP |
|
Rosiglitazone |
122320-73-4 |
USP30 |
|
Esomeprazole Magnesium |
161973-10-0 |
USP/BP |
|
Topiramate |
97240-79-4 |
USP31 |
|
Fexofenadine HCl |
153439-40-8 |
Inhouse |
|
Bosentan |
147536-97-8 |
Inhouse |
|
D-Cysteine |
921-01-7 |
Inhouse |
|
D-Phenylalanine |
673-06-3 |
Inhouse |
|
Linagliptin |
668270-12-0 |
Inhouse |
|
Rivaroxaban |
366789-02-8 |
USP |
|
Saxagliptin |
361442-04-8 |
USP |
|
Vildagliptin |
274901-16-5 |
USP |
Major Pharmaceutical Intermediates
| Items Descripation | Structure | Application |
|
MICA ESTER CAS No: 246035-38-1 Purity: ≥98% |
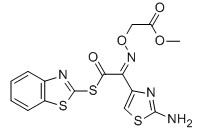 |
For Cefixime |
|
EHATA CAS No: 64485-82-1 Purity: ≥98% |
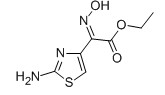 |
For Ceftazidine |
|
2-Chloroadenine CAS No: 1839-18-5 |
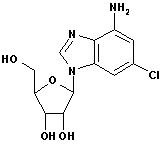 |
For Cladribine, Fludarabine et al |
|
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide CAS No: 14805o-29-9 |
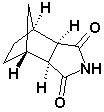 |
For Lurasidne |
|
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane CAS No: 186204-35-3 |
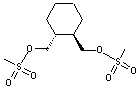 |
For Lurasidone |
|
3-(Piperazin-1-yl)benzol[d] isothiazole CAS No: 87691-87-0 |
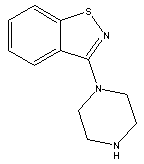 |
For Lurasidone |
|
Trityl olmesartan CAS No: 144690-92-6 Purity: ≥98% |
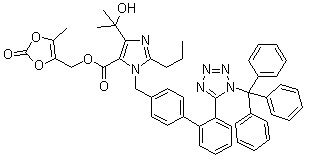
|
For olmesartan |
|
3-Acetyl Pyridine CAS No: 350-03-8 |
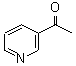
|
For Risedronate Sodium |
|
3-(AceticAcid)pyridine HCL CAS No: 6419-36-9 |
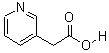 |
For Risedronate Sodium |
|
Risedronic Acid CAS No: 105462-24-6 |
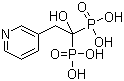 |
For Risedronate Sodium |
|
3-Hydroxy-1-adamantyl-D-Glycine CAS No: 709031-29-8 |
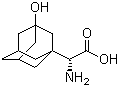 |
For Saxagliptin |
|
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester CAS No: 361440-67-7 |
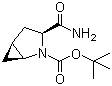 |
For Saxagliptin |
|
(S)-N-Boc-3- hydroxy-adamantylglycine CAS No: 361442-00-4 |
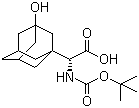 |
For Saxagliptin |
|
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)- CAS No: 866083-42-3 |
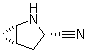 |
For Saxagliptin |
|
Ethyl 3-(pyridin-2-ylamino) propanoate CAS No: 103041-38-9 |
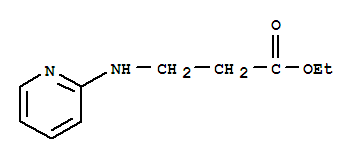 |
For Dabigatran |
|
N-(4-Cyanophenyl) glycine CAS No: 42288-26-6 |
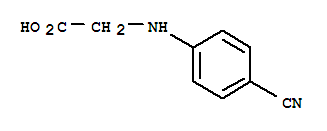 |
For Dabigatran |
|
4-methylamino-3-nitrobenzoic Acid CAS No: 41263-74-5 |
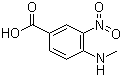 |
For Dabigatran |
|
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL CAS No: 167834-24-4 |
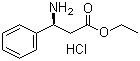 |
For Dapoxetine |
|
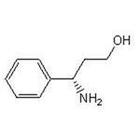
(S)-3-Amino-3-Phemylpropan -1-ol CAS No: 82769-76-4 |
|
For Dapoxetine |
|
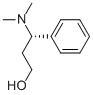
(S)-3-Dimethylamino-3-Phemylpropanol CAS No: 82769-75-3 |
|
For Dapoxetine |
|
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid CAS No: 832088-68-3 |
For Fexofenadine HCl | |
|
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate CAS No:154477-54-0 |
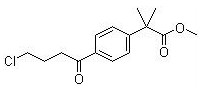
|
For Fexofenadine HCl |
|
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone CAS No 461432-22-4 |
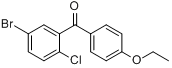
|
For Dapagliflozin |
|
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether CAS No :461432-23-5 |
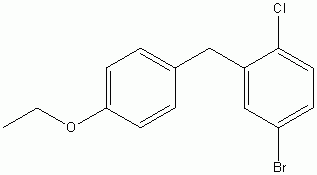
|
For Dapagliflozin |
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.pharma-voice.com